January 9, 2011
Appropriate Use Criteria for Cardiac CT (2010)
August 15, 2010
Pulmonic Valvular Stenosis
- Common congenital heart defects, approximately 10% of all cases
- Classified as subvalvular, valvular and supravalvular stenosis (based on level of obstruction) and as mild, moderate and severe (based on pressure gradient across stenosis). It can occur in branch pulmonary arteries as well
- May occur in isolation (as in our case) or be associated with other complex congenital heart defects
- In severe cases, physical and ECG findings of right axis deviation, right ventricular hypertrophy would be apparent
- Cardiac ultrasound: obstruction at right ventricular outflow tract (RVOT), pulmonary valve (PV), main pulmonary artery, right and left pulmonary arteries, abnormal pulmonary valve annulus, abnormal pressure gradients across RVOT, PV and pulmonary arteries
- Radiography: enlargement of the main pulmonary artery, right ventricular hypertrophy. Radiographic differential diagnoses are pulmonary hypertension, idiopathic dilatation of the pulmonary trunk
July 15, 2010
Anatomic Position of Heart Valves


- The three heart valves (aortic, mitral and tricuspid) commonly overlap each other on frontal radiograph. Correct radiographic identification can be difficult.
- To differentiate the mitral from aortic valve on lateral view, one draws a line from the junction of the sternum and diaphragm to the carina. This line normally intersects aortic valve*. The valve below the line is mitral valve. The tricuspid valve is the one to the mitral valve.
- Without a lateral view, the best criterion for use in differentiating between aortic and mitral prostheses is the direction of flow (discernable in Starr-Edwards and most Bjork-Shiley prostheses). Orifice (en face or in profile) and orientation (vertical or horizontal) of prosthesis are less reliable.
June 15, 2010
Left Atrial Enlargement

- Convex left atrial appendage
- Double density on the right side of the spine (one of the earliest signs)
- Double density on the left side as the left atrium extends into the left lower lobe
- Posterior displacement of the left mainstem bronchus posteriorly on lateral view, and superiorly on frontal view
- Spreading of the carina
- Acquired: mitral valve disease (stenosis or regurgitation), left ventricular failure, left atrial myxoma
- Congenital: VSD, PDA, hypoplastic left heart complex
March 15, 2010
Kartagener Syndrome
 Figure 1: Frontal chest radiograph demonstrates dextrocardia (cardiac apex pointing to the right with right aortic arch - arrowheads), bibasilar coarse reticular opacities and loss of volumes in the lower lungs in a patient with Kartagener syndrome.
Figure 1: Frontal chest radiograph demonstrates dextrocardia (cardiac apex pointing to the right with right aortic arch - arrowheads), bibasilar coarse reticular opacities and loss of volumes in the lower lungs in a patient with Kartagener syndrome. Figure 2: Coronal reformatted CT image confirms the presence of extensive bibasilar bronchiectasis (arrows) and situs inversus. Bronchiectasis in patients with this syndrome tends to involve the dependent parts of the lungs.
Figure 2: Coronal reformatted CT image confirms the presence of extensive bibasilar bronchiectasis (arrows) and situs inversus. Bronchiectasis in patients with this syndrome tends to involve the dependent parts of the lungs.
- Genetically transmitted (autosomal recessive) syndrome characterized by bronchiectasis, situs inversus and chronic sinusitis
- Genetic disorder first described in 1904, but identified as a syndrome by Manes Kartagener, a Swiss internist, in 1933
- It is a subtype of primary cilia dyskinesia
- Incidence 1:32000 live births
- Abnormal function of cilia is believed to be responsible for visceral asymmetry (abnormal movement of cilia in certain embryonic epithelial tissues), respiratory disease, etc.
- Onset of upper and lower respiratory tract symptoms shortly after birth in the presence of situs inversus
- Family history of primary ciliary dyskinesia or Kartagener syndrome
- Confirmation with biopsy of respiratory mucosa or microscopic examination of sperms
- Other clinical signs: chronic rhinitis with nasal polyposis, agenesis of frontal sinuses, repeated otitis media, bronchiectasis (usually dependent parts of the lungs in contrast to cystic fibrosis that tends to affect the upper lobes), situs inversus (complete or partial)
February 24, 2010
Pericardial Effusion - Oreo Cookie Sign
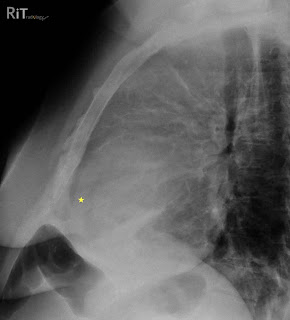 Figure 1: Lateral chest radiograph shows separation of the retrosternal (dark line parallel to the sternum anterior to the yellow star) and epicardial fat stripes (dark line behind the yellow star). This patient also has an anterior mediastinal mass due to lymphoma.
Figure 1: Lateral chest radiograph shows separation of the retrosternal (dark line parallel to the sternum anterior to the yellow star) and epicardial fat stripes (dark line behind the yellow star). This patient also has an anterior mediastinal mass due to lymphoma. Figure 2: Axial contrast-enhanced CT image shows a large pericardial effusion (stars) separating the retrosternal fat stripe (double-headed arrow) and epicardial fat stripe (arrowheads).
Figure 2: Axial contrast-enhanced CT image shows a large pericardial effusion (stars) separating the retrosternal fat stripe (double-headed arrow) and epicardial fat stripe (arrowheads).
- Pericardium has two layers: visceral (attached to myocardial surface and proximal great vessels) and parietal (free wall of pericardial sac)
- Pericardial sac normally contains 20-50 mL of fluid
- Most common cause = myocardial infarction with left heart failure
- Other causes: uremia, hypoalbuminemia, myxedema, infection, drug reaction, trauma, neoplasm, autoimmune disease
- Can be seen on radiography if volume exceeds 250 mL
- PA or AP radiograph: water bottle-shaped morphology of the cardiomediastinal shadow
- Lateral view: separation of retrosternal and epicardial fat stripes by more than 2 mm (Oreo cookie sign)
- Oreo cookie sign: epicardial fat and retrosternal fat stripes = outer dark cookie layers; opaque fluid = white fluff of the cookie
Parker MS, Chasen MH, Paul N. Radiologic signs in thoracic imaging: case-based review and self-assessment module. AJR 2009;192:S34-S48.
January 15, 2010
Ectopic Origin of Right Coronary Artery
 Axial CT image shows an ectopic origin of the right coronary artery (arrow) arising from the left cusp and passes between the ascending aorta and the right ventricular outflow tract in the "intraseptal" course.
Axial CT image shows an ectopic origin of the right coronary artery (arrow) arising from the left cusp and passes between the ascending aorta and the right ventricular outflow tract in the "intraseptal" course.
- 1% of all patients undergoing cardiac catheterization
- Three types: ectopic origin from a coronary cusp (like our case), absent coronary artery, ectopic origin from a main pulmonary artery
- 20% causes life threatening symptoms such as arrhythmias, syncope, myocardial infarction or sudden death
- MDCT can clearly show the origin and course of several forms of anomalous coronary artery.
- Either left coronary originating from the right cusp (sinus) or right coronary arising from the left cusp (sinus), if it courses between the aorta and MPA -- it is called "intervascular course" and is associated with poor outcome.
- If it courses between the aorta and RV outflow tract inferior to the plane of the pulmonic valve, it is "intraseptal course" and considered "comparatively benign"
September 15, 2009
Bicuspid Aortic Valve
 Fig. 1: Chest radiograph of a 44-year-old man shows prominence of the right cardiac contour, probably due to enlarged ascending aorta and aortic root. The overall heart size is slightly increased.
Fig. 1: Chest radiograph of a 44-year-old man shows prominence of the right cardiac contour, probably due to enlarged ascending aorta and aortic root. The overall heart size is slightly increased. Fig. 2: Axial CT image at the level of the aortic valve shows a bicuspid valve (arrows) with calcifications of the valve leaflets (arrowheads).
Fig. 2: Axial CT image at the level of the aortic valve shows a bicuspid valve (arrows) with calcifications of the valve leaflets (arrowheads).
Facts
- Most common congenital cardiac malformation, 1%-2% of population
- Majority of cases develop complications requiring treatment
- Abnormal cusp formation during valvulogenesis
- May be a part of a continuum: unicuspid valve (severe form), bicuspid valve (moderate form), tricuspid valve (normal) and quadricuspid form
- May be genetic. Not clear if inheritable but studies had shown that this condition could be transmitted in autosomal dominance form, with male to female ratio of 4:1.
- Most common = aortic stenosis
- Bicuspid aortic valve very common among patients age 15-65 years with significant aortic stenosis
- The fewer the number of cusps, the greater is the chance that the valve is stenotic from birth
- Other complications: aortic insufficiency, endocarditis, aortic dilatation/aneurysm, dissection
- It is believed that changes of the aorta (dilation, aneurysm) are not secondary to valvular dysfunction, but rather a manifestation of the disease itself
Surgery if: Severe aortic insufficiency/stenosis, dilated aorta, increased left ventricular size, decreased left ventricular function
Our case: bicuspid aortic valve with aortic stenosis.
Reference:
Fedak PW, Verma S, David TE, et al. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002;106:900.
August 15, 2009
Mitral Annulus Calcification
- Noninflammatory chronic degenerative process
- Mitral valve ring (annulus) may calcify in older individuals (>60 years)
- Women more common than men
- Small calcium forms in or below mitral annulus at junction between ventricular myocardium and posterior mitral leaflet
- Severe disease resembles the letter J, O, or reversed C
- Little clinical significance in most cases
- May grow into ventricular myocardium to produce heart block, into mitral leaflets to cause mitral regurgitation and stenosis, through endocardium to cause small systemic emboli
- Mitral annulus calcification in the elderly is an independent risk factor of developing stroke
July 15, 2009
Lipomatous Hypertrophy of Interatrial Septum
 Figure: Axial chest CT image (non cardiac-gating) shows a fatty mass (arrows) in the interatrial septum with a "dumbbell shape" sparing the fossa ovalis (double-headed arrow). The mass extends to the wall of the superior vena cava.
Figure: Axial chest CT image (non cardiac-gating) shows a fatty mass (arrows) in the interatrial septum with a "dumbbell shape" sparing the fossa ovalis (double-headed arrow). The mass extends to the wall of the superior vena cava.
- Excessive deposition of fat in the interatrial septum of unknown etiology
- Not common, incidence about 1% in autopsy, 2% on CT and up to 8% on echocardiography
- In most cases, they are incidental. However, they may cause arrhythmias
- Fatty mass with thickness >/= 20 mm, sharp margin, no enhancement
- Spares fossa ovalis, resulting in a "dumbbell" shape on axial images
- Fat can extend to the level of coronary sinus and to the aortic root
- Atrial myxoma: this tumor arises from fossa ovalis and is usually pedunculated
- Cardiac lipoma: encapsulated, true neoplasm in younger patients
February 16, 2009
Subendocardial Fat in Myocardial Infarction
 Fig: Axial contrast-enhanced CT image shows a curvilinear low attenuation in the subendocardial portion of the left anterior descending (LAD) coronary artery territory of the left ventricle. The patient had a history of remote myocardial infarction (MI).
Fig: Axial contrast-enhanced CT image shows a curvilinear low attenuation in the subendocardial portion of the left anterior descending (LAD) coronary artery territory of the left ventricle. The patient had a history of remote myocardial infarction (MI).
Myocardial Fat
- Common entity, found histologically in 68% of ischemic heart disease patients and 84% of patients with history of MI.
- May result from inability of ischemic myocytes to metabolize fatty acids.
- Can be shown on cardiac MRI and CT.
- In one study, it was detected in 22% of cases and it was most commonly seen in LAD territory.
What does it mean?
- It is associated with greater infarct age
- Patients with myocardial fat had more severe regional wall motion abnormalities on echocardiography
Ahn SS, et al. CT detection of subendocardial fat in myocardial infarction. AJR 2009;192:532-537.
October 16, 2008
Pericardial Effusion on Lateral Chest Radiograph
 Fig. 1. Lateral chest radiograph shows small bilateral pleural effusions. Enlarged cardiac silhouette with abnormal bands of density noted anterior to the heart (between arrows). Of note, this band of density consists of a central soft tissue sandwiched by lucent stripes.
Fig. 1. Lateral chest radiograph shows small bilateral pleural effusions. Enlarged cardiac silhouette with abnormal bands of density noted anterior to the heart (between arrows). Of note, this band of density consists of a central soft tissue sandwiched by lucent stripes. Fig. 2. Sagital CT image of the same patient correlates well with lateral radiograph in Fig. 1. It shows that the 'sandwich' seen anterior to the heart represents pericardial effusion (star) bounded by epicardial and pericardial fat.
Fig. 2. Sagital CT image of the same patient correlates well with lateral radiograph in Fig. 1. It shows that the 'sandwich' seen anterior to the heart represents pericardial effusion (star) bounded by epicardial and pericardial fat.
Facts
- Pericardial effusion can be transudate or exudate (pus, blood, infection)
- Symptoms depend on the size and rate of accumulation of effusion
- Chest radiography is not diagnostic of pericardial effusion in most cases
- CT and MRI used to assess size and extent of pericardial effusion
- Measurement of pericardial effusion by CT/MRI tends to be larger than in echocardiography
All four signs are sensitive (71-100%) but not specific (12-46%).
- Enlarged cardiac silhouette with sharp margin, "water bottle" silhouette
- Pericardial fat stripe (separation of pericardial layers)
- Predominantly left-sided pleural effusion
- Increased transverse cardiac diameter compared with previous radiograph
Listen to podcast HERE (Thai language).
Reference:
1. Eisenberg MJ, et al. Diagnostic value of chest radiography for pericardial effusion. J Am Coll Cardiol 1993; 22:588-593
2. Guidelines on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587-610
August 11, 2008
Three 'Reasonable and Appropriate' Indications for Coronary CTA for Detection of CAD
 กล่าวตามบรรยายของ Dr. Mamuya ซึ่งเป็น MGH cardiologist รับเชิญมาพูดเกี่ยวกับ Clinical Role of Cardiac CTA ที่เพิ่งผ่านมาเร็วๆ นี้
กล่าวตามบรรยายของ Dr. Mamuya ซึ่งเป็น MGH cardiologist รับเชิญมาพูดเกี่ยวกับ Clinical Role of Cardiac CTA ที่เพิ่งผ่านมาเร็วๆ นี้
Indications ที่เหมาะสมในการทำ Coronary CTA for detection of CAD มี 3 ข้อ
- Symptomatic patients with intermediate pretest probability who have either uninterpretable EKG or unremarkable EKG
- Symptomatic patients with uninterpretable stress test
- Symptomatic patients with new onset of heart failure
สรุปว่า
Coronary CTA is appropriate in SYMPTOMATIC patients with INTERMEDIATE pretest probability
Reference:
Hendel, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR Appropriateness Criteria for Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging. JACC (2006)
July 27, 2008
Clinical Role of Cardiac CTA (2008)

จากบรรยายของ Dr. Mamuya ได้สรุปไว้เป็นหัวข้อสั้นๆ ถึงบทบาทของ Cardiac CTA ในขณะนี้ที่ถือว่า ได้เดินทางมาพอควร แต่ยังไม่ไกลมากเนื่องจากมีข้อจำกัดทางเทคนิก และยังขาดข้อมูลที่น่าเชื่อถือมาสนับสนุน
Cardiac Anatomy: Coronary anomalies, congenital cardiac anomalies
Detection of CAD: Symptomatic patients with intermediate risk
Stent Evaluation: Good for stent size > 3 - 3.5 mm
Dilated Cardiomyopathy: to rule out ischemic cause
Prior to Non-coronary Cardiac Surgery (screen for status of coronary artery)
อย่าลืมว่าข้อจำกัดหลายๆ อย่าง และ study ใหม่ๆ ที่ออกมาในวงการ อาจส่งผลให้บทบาทของ cardiac CTA กว้างขวางและชัดเจนมากขึ้นครับ
July 6, 2008
ASD with PAPVR
 รูป 1 - Chest x-ray ของผู้ป่วยหญิงอายุ 20 ปี แสดงให้เห็นลักษณะของ increased pulmonary vasculature (shunt vascularity) ซึ่งพบได้ใน ASD, VSD, PDA หรือ PAPVR
รูป 1 - Chest x-ray ของผู้ป่วยหญิงอายุ 20 ปี แสดงให้เห็นลักษณะของ increased pulmonary vasculature (shunt vascularity) ซึ่งพบได้ใน ASD, VSD, PDA หรือ PAPVR รูป 2 - Axial CT แสดงให้เห็น Right upper lobe pulmonary vein (yellow arrow) draining into SVC, เข้าได้กับ Partial Anomalous Pulmonary Venous Return (PAPVR).
รูป 2 - Axial CT แสดงให้เห็น Right upper lobe pulmonary vein (yellow arrow) draining into SVC, เข้าได้กับ Partial Anomalous Pulmonary Venous Return (PAPVR). รูป 3 - Cine MR of the heart (4-chamber view) แสดงให้เห็น atrial septal defect (red arrow) ชนิด sinus venosus.
รูป 3 - Cine MR of the heart (4-chamber view) แสดงให้เห็น atrial septal defect (red arrow) ชนิด sinus venosus. รูป 4 - Diagram of sinus venosus ASD (from Mayoclinic.com)
รูป 4 - Diagram of sinus venosus ASD (from Mayoclinic.com)
Atrial septal defect (ASD)
- พบประมาณ 30% ของ congenital heart disease ที่วินิจฉัยในผู้ใหญ่
- ส่วนมากเกิดจาก spontaneous genetic mutation
- มี 4 ชนิด คือ ostium secondum (most common), ostium primum, sinus venosus, coronary sinus (least common)
- Pathophysiology ขึ้นกับขนาดของ ASD, ถ้าขนาดใหญ่อาจทำให้เกิด left to right shunt, pulmonary hypertension, right ventricular failure.
- CXR: prominent pulmonary vasculature, RA and RV enlargement, PA dilatation (ในคนไข้ที่มี hemodynamically significant shunting)
- วินิจฉัยได้จาก echocardiography โดย secondum and primum defects เห็นได้โดย transthoracic echocardiography. ส่วน sinus venosus defect เห็นได้ดีกว่าด้วย transesophageal technique.
- Sinus venosus ASD มักพบที่ junction ของ superior vena cava
- Sinus venosus ASD มักพบร่วมกับ PAPVR
Reference:
McCormick DJ. Atrial Septal Defect: Pathophysiology, Diagnosis, and Treatment. Medscape Radiology
May 20, 2008
Left Ventricular Mass in Patient with Old Myocardial Infarction
 ภาพ 1 - axial CT with IV contrast แสดงให้เห็น thinning of the apical and septal wall และ fat density ใน subendocardium บริเวณเดียวกัน (ลูกศรเหลือง).
ภาพ 1 - axial CT with IV contrast แสดงให้เห็น thinning of the apical and septal wall และ fat density ใน subendocardium บริเวณเดียวกัน (ลูกศรเหลือง). ภาพ 2 - axial CT รูปล่างลงมาพบว่ามี filling defect (ลูกศรแดง) ที่บริเวณ apex ของ left ventricle
ภาพ 2 - axial CT รูปล่างลงมาพบว่ามี filling defect (ลูกศรแดง) ที่บริเวณ apex ของ left ventricle
สาเหตุของ cardiac mass มีหลายอย่าง เช่น thrombus, tumor (primary or metastasis).
Diagnosis: LV thrombus with old myocardial infarction.
ข้อควรรู้
- Cardiac thrombi เป็น most common cause of cardiac mass
- พบได้ 5% - 23% หลังเกิด acute MI
- เสี่ยงต่อการเกิด systemic embolization
- ผู้ป่วยที่มีความเสี่ย่งในการเกิด cardiac thrombus เช่น ผู้ที่มี abnormal endocardium, loss of atrial contraction, arrhythmia, chamber dilatation และ impaired LV function
Rehan A, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound (Apr 2006)

















